A crystalline molecular gyrotop with a biphenylene dirotor and its temperature-dependent birefringence†
Abstract
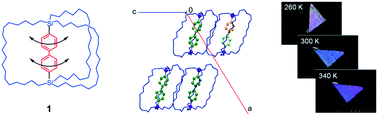
Biphenyl contains a direct bond between two phenyl functional groups, and the dihedral angle between the two phenyl groups has been discussed in terms of electronic and steric stabilization. Facile rotation about the C–C bond between the two phenyl components is expected to occur at high temperatures. Here, we report observations of the rotational dynamics of a biphenylene dirotor in a crystalline state in a novel molecular gyrotop. The structure and rotational dynamics of the rotor exhibit remarkable temperature dependence. In accordance with the amplification of the angular displacement of biphenylene inside a crystal with increasing temperature, the birefringence of the single crystal decreased.


 Please wait while we load your content...
Please wait while we load your content...