The first anion-exchange membrane fuel cell to exceed 1 W cm−2 at 70 °C with a non-Pt-group (O2) cathode†
Abstract
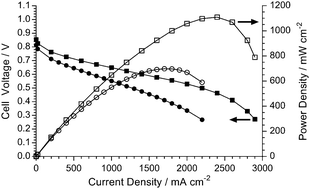
Anion-exchange membrane fuel cells face two challenges: performance and durability. Addressing the first, we demonstrate high performance with both O2 and CO2-free air supplies, even when using a Ag/C cathode. This was enabled by the development of a radiation-grafted anion-exchange membrane that was less than 30 μm thick when hydrated.


 Please wait while we load your content...
Please wait while we load your content...