Synthesis of seminaphtho-phospha-fluorescein dyes based on the consecutive arylation of aryldichlorophosphines†
Abstract
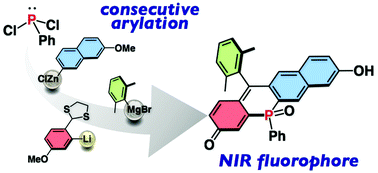
Seminaphtho-phospha-fluorescein (SNAPF), a phosphine-oxide-containing unsymmetric fluorescein dye, was synthesized based on the consecutive arylation of PhPCl2, followed by Friedel–Crafts cyclization. The resulting SNAPF exhibited several attractive photophysical properties including an intense fluorescence in the NIR region and a large Stokes shift.


 Please wait while we load your content...
Please wait while we load your content...