Reversible formation and cleavage of Pt→Ag dative bonds in a pre-organized cavity of a luminescent heteropolynuclear platinum(ii) complex†
Abstract
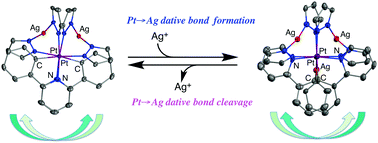
A U-shaped Pt2Ag2 complex [Pt2Ag2(ppy)2(Ph2pz)4] with a pre-organized cavity (ppy = 2-phenylpridinate and Ph2pz = 3,5-diphenylpyrazolate) and related complexes have been prepared. The Pt2Ag2 complexes react with Ag(I) ions to give the corresponding Pt2Ag3 complexes containing Pt→Ag dative bonds. It became obvious that the existence of the C(ipso) atom in the chelate ligand is important as the driving force for forming Pt→Ag dative bonds. However, once the Pt2Ag3 complex is formed, the trapped Ag(I) ion is mainly stabilized by the Pt→Ag dative bonds, which are stronger than the Ag–C(ipso) bond. The trapped Ag(I) ion can be abstracted from the cavity selectively by adding an equivalent amount of chloride ion into the solution of Pt2Ag3 complexes to reproduce the original Pt2Ag2 complexes.


 Please wait while we load your content...
Please wait while we load your content...