A deciduous directing group approach for the addition of aryl and vinyl nucleophiles to maleimides†
Abstract
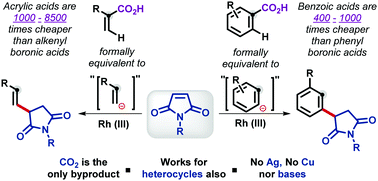
A Rh(III)-catalyzed C–H activation followed by conjugate addition to maleimides, using carboxylic acid as a traceless/deciduous directing group, to formally furnish a Csp2–Csp3 bond is presented. For the first time, derivatives of acrylic acid have been shown as surrogates for the unstable and expensive alkenyl boronic acids.


 Please wait while we load your content...
Please wait while we load your content...