Revisiting the Juliá–Colonna enantioselective epoxidation: supramolecular catalysis in water†
Abstract
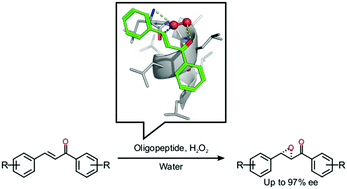
We describe an efficient epoxidation process leading to chiral epoxyketones using the reusable homo-oligopeptide poly-L-leucine (PLL) in pure water, without any organic co-solvent. A range of substituted epoxyketones can be accessed with good conversions and high enantioselectivities. Based on the experimental results and computational studies, we propose a mechanism that demonstrates the importance of both the α-helical structure and the presence of a hydrophobic groove of the homo-oligopeptide catalyst for reactivity and selectivity.
- This article is part of the themed collection: CSC100: Celebrating Canadian Chemistry


 Please wait while we load your content...
Please wait while we load your content...