PtCl4-catalyzed skeleton rearrangement–cyclization of tertiary indolyl-3-alkynols†
Abstract
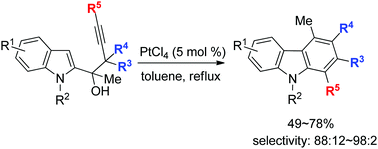
An unexpected PtCl4-catalyzed cyclization reaction of tertiary indol-2-yl-3-alkynols occurred smoothly in toluene to form various poly-substituted carbazole derivatives efficiently. A possible mechanism involving the formation of a spiro-tricyclic intermediate via indole C-2 attack, highly selective ring expansion and then 1,2-migration has been proposed for the formation of carbazoles.


 Please wait while we load your content...
Please wait while we load your content...