N–N bond formation in Ugi processes: from nitric acid to libraries of nitramines†
Abstract
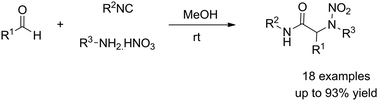
The Ugi reaction has drawn considerable attention over the years leading to numerous libraries of heterocycles and various extensions changing the nature of the components of the coupling. We report here the use of nitric acid as carboxylic acids surrogates, displaying the first aminative Ugi-type reaction leading to nitramines.


 Please wait while we load your content...
Please wait while we load your content...