The metalation of hen egg white lysozyme impacts protein stability as shown by ion mobility mass spectrometry, differential scanning calorimetry, and X-ray crystallography†
Abstract
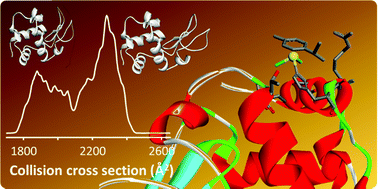
Metalation of hen egg white lysozyme (HEWL) with organometallics was studied with physicochemical methods in solid state, solution and the gas phase. While metalation did not affect the crystal structure of HEWL significantly, protein destabilisation was detected in gas phase and solution.


 Please wait while we load your content...
Please wait while we load your content...