Silver-catalysed tandem hydroamination and cyclization of 2-trifluoromethyl-1,3-enynes with primary amines: modular entry to 4-trifluoromethyl-3-pyrrolines†
Abstract
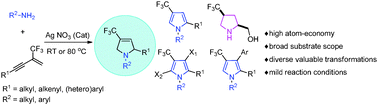
A highly efficient tandem hydroamination and cyclization reaction of 2-trifluoromethyl-1,3-enynes with primary amines leading to 4-trifluoromethyl-3-pyrrolines was developed by using AgNO3 as a catalyst under mild reaction conditions. This new method is compatible with alkyl, aryl, and allyl primary amines, representing an atom-economical protocol for the construction of 4-trifluoromethyl-3-pyrrolines for the first time.


 Please wait while we load your content...
Please wait while we load your content...