UVB exposure enhanced the dermal penetration of zinc oxide nanoparticles and induced inflammatory responses through oxidative stress mediated by MAPKs and NF-κB signaling in SKH-1 hairless mouse skin†
Abstract
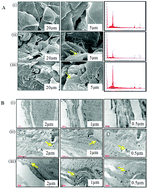
Besides titanium dioxide (TiO2), zinc oxide (ZnO) nanoparticles (NPs) are commonly used in sunscreen formulations as protective agents against exposure to ultraviolet radiation. Although the majority of prior studies have concluded that NPs do not penetrate healthy skin, compromised skin slightly enhanced metal oxide NP penetration. However, a question arises regarding the possible toxic consequences if consumers who had applied sunscreens containing ZnO-NPs were exposed to environmentally relevant doses of UVB. Considering this, we planned a study where SKH-1 hairless mice were topically exposed to a 5% and/or 10% dose of ZnO-NPs (<50 nm and <100 nm) either alone or along with UVB (50 mJ cm−2). In two additional groups, mice were treated with either bulk ZnO-NPs (<5 μm) or with ZnO-NPs (<5 μm) and subsequently UVB (50 mJ cm−2). Animals of all groups were sacrificed after 6, 24 and 48 h and the Zn ion content in the skin was measured. In addition, estimation of ROS generation, histopathology, myeloperoxidase (MPO) activity, immunohistochemistry for COX-2 and western blot analysis for MAPKs, p-IκBα, p-NF-κB, and COX-2 were also carried out. Significant increases in the Zn ion in exposed skin were seen. Enhanced ROS generation and MPO activity were also found in ZnO-NPs followed by UVB exposed groups at all three time points. Similarly, hyperplasia and over-expression of COX-2 were also greater in ZnO-NPs and UVB exposed groups than in the ZnO-NPs and UVB only groups. The expression of MAPKs, and transcription factors NF-κB along with COX-2 were also enhanced significantly in ZnO-NPs and the UVB treated group. Collectively, our findings suggest that UVB exposure enhanced ZnO-NP penetration in mouse skin and possibly dissolution of these ZnO-NPs takes place during this process, causing significant Zn ion generation leading to oxidative stress by ROS generation which subsequently activates MAPK-NF-κB signaling and increases COX-2 and inflammation.

 Please wait while we load your content...
Please wait while we load your content...