Flexible and highly fluorescent aromatic polyimide: design, synthesis, properties, and mechanism†
Abstract
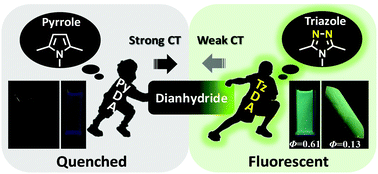
To develop high-performance flexible fluorescent aromatic polyimides and to have a deeper insight into the fluorescence mechanism, two diamine monomers PyDA and TzDA with similar chemical structures but totally different electronic effects, were designed and synthesized. PyDA bears an electron-donor pyrrole group while TzDA bears an electron-acceptor 1,2,4-triazole group. The resulting aromatic polyimide TzODPI containing the triazole group showed bright green photoluminescence with a high quantum yield of up to 61% in solution and 13% in the film state. However, fluorescence was totally quenched in the pyrrole-containing polyimide PyODPI. The completely different phenomena were systematically elucidated with the aid of molecular simulations. Theoretical calculations for the molecular orbital distribution, oscillator strength, and the electron transition process between the ground state and excited state of model molecules were applied to clarify the fluorescence mechanism. Highly fluorescent aromatic polyimides can be obtained by appropriate control of the intra-molecular charge-transfer effects between the diamine and dianhydride moieties, and this demonstrates a simple strategy to develop wholly aromatic polyimides with high fluorescence for potential applications in the field of flexible displays.


 Please wait while we load your content...
Please wait while we load your content...