Effects of PNDIT2 end groups on aggregation, thin film structure, alignment and electron transport in field-effect transistors†
Abstract
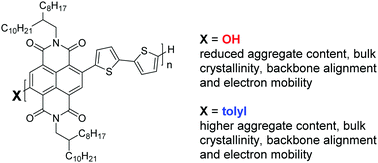
To develop greener protocols toward the sustainable production of conjugated polymers, we combine the advantages of atom-economic direct arylation polycondensation (DAP) with those of the green solvent 2-methyltetrahydrofuran (MeTHF). The n-type copolymer PNDIT2 is synthesized from unsubstituted bithiophene (T2) and 2,6-dibromonapthalene diimide (NDIBr2) under simple DAP conditions in MeTHF. Extensive optimization is required to suppress nucleophilic substitution of NDIBr end groups, which severely limits molar mass. Different carboxylic acids, bases, palladium precursors and ligands are successfully screened to enable quantitative yield and satisfyingly high molar masses up to Mn,SEC ∼ 20 kDa. In contrast to PNDIT2 made via DAP in toluene with tolyl-chain termini, nucleophilic substitution of NDIBr chain ends in MeTHF finally leads to NDI-OH termination. The influence of different chain termini on the optical, thermal, structural and electronic properties of PNDIT2 is investigated. For samples with identical molecular weight, OH-termination leads to slightly reduced aggregation in solution and bulk crystallinity, a decreased degree of alignment in directionally deposited films, and a consequently reduced, but not compromised, electron mobility with promising values still close to 0.9 cm2 V−1 s−1.

 Please wait while we load your content...
Please wait while we load your content...