Formation mechanism of graphene quantum dots and their edge state conversion probed by photoluminescence and Raman spectroscopy†
Abstract
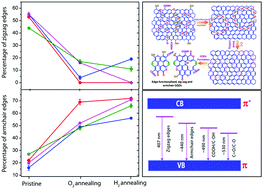
We investigate the formation mechanism of graphene quantum dots (GQDs) from a graphene oxide (GO) precursor and study the inter-conversion of edge states during thermal annealing of GQDs through various microscopic and spectroscopic tools. Monitoring the early stages of growth of GQDs reveals that the in-plane epoxy (C–O–C) functional groups attached at the defect sites essentially cut the oxidized GOs into small pieces of GQDs during the hydrothermal reaction. By conducting a series of controlled annealing experiments under oxygen and hydrogen gas environments, we monitor and quantify the inter-conversion of edge states and functional groups in GQDs from Raman and photoluminescence (PL) studies. The deconvolution of the Raman spectrum allowed us to monitor the evolution of new Raman bands at ∼1260 and ∼1438 cm−1, which are assigned to the edge functional groups. Through the fitting of the PL spectra and a quantitative analysis of integrated intensities of PL, we unambiguously assign the blue PL emission bands at ∼407 and ∼440 nm to zigzag and armchair free edge states in GQDs, respectively, for the first time. On the other hand, the green emission bands at ∼490 and ∼530 nm are attributed to COOH/C–OH and C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O/C–O edge functional groups, respectively. Our conclusions are corroborated by XPS and FTIR spectroscopic analyses of the as-grown and annealed GQDs.
O/C–O edge functional groups, respectively. Our conclusions are corroborated by XPS and FTIR spectroscopic analyses of the as-grown and annealed GQDs.

 Please wait while we load your content...
Please wait while we load your content...