Self-assembly and mechanochromic luminescence switching of trifluoromethyl substituted 1,3,4-oxadiazole derivatives†
Abstract
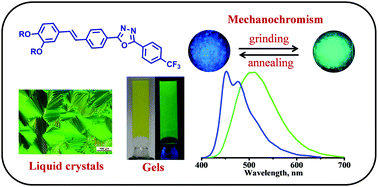
The synthesis, self-assembly and mechanochromic properties of a series of oxadiazole derivatives (TFOXD1, TFOXD4, TFOXD6 and TFOXD8) substituted with a dialkoxy stilbene and trifluoromethyl benzene moieties are reported. All the derivatives formed stable gels in aliphatic solvents and with the exception of TFOXD1 exhibited monotropic liquid crystalline behavior. The photophysical properties of their films and gels were dependent on the length of their alkoxy substituent, with the formation of excimers being observed in the derivatives containing longer alkoxy chains. TFOXD4 and TFOXD6 exhibited reversible mechanochromic luminescence switching. XRD studies confirmed the reversible phase transformation between crystalline and amorphous forms.

 Please wait while we load your content...
Please wait while we load your content...