The effect of N-alkyl chain length on the photophysical properties of indene-1,3-dionemethylene-1,4-dihydropyridine derivatives†
Abstract
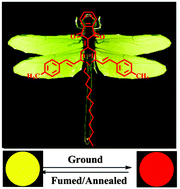
A series of donor–π–acceptor indene-1,3-dionemethylene-1,4-dihydropyridine (IDM-DHP) derivatives with different alkyl chain lengths on the DHP ring were synthesized to investigate the effect of N-alkyl chains on their fluorescence properties in the aggregated state. These compounds with highly distorted conformations show obvious aggregation-induced emission enhancement phenomena in a THF/water mixture because of the restriction of intramolecular rotation in the aggregated state, as confirmed by solution thickening experiments. The as-synthesized IDM-DHP solids emit strong yellow fluorescence and the general trend of their emission wavelengths decreases with the increase of the N-alkyl chain length. It is found that these compounds exhibit outstanding reversible mechanofluorochromic (MFC) properties, and N-alkyl chains play a functional role in tuning their MFC behaviours, that is, the longer alkyl-containing IDM-DHP solids show larger MFC spectral redshifts, which should be ascribed to the weaker CH/π hydrogen bonds in the molecules and as a result looser molecular stacking, as revealed by the single crystal X-ray diffraction analysis. Additionally, the fluorescence emission of the ground samples of these compounds can recover by annealing or solvent fuming. X-ray diffraction experiments reveal that the transformation between crystalline and amorphous states under various external stimuli should be responsible for the MFC properties. This work demonstrates that a simple alkyl chain change in the skeleton of some organic fluorophores can be used to tune their fluorescence properties in the aggregated state, which provides good design ideas for the development of novel IDM-DHP derivatives with MFC properties.

 Please wait while we load your content...
Please wait while we load your content...