Trend breaking substitution pattern of phenothiazine with acceptors as a rational design platform for blue emitters†
Abstract
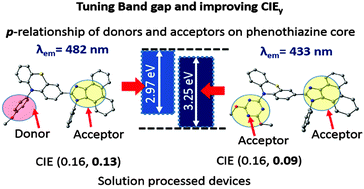
To investigate the effect of unusual substitutions in the phenothiazine core toward the development of deep blue emitters, two compounds have been designed and synthesized by integrating electron donor and electron acceptor units in a nonconventional fashion. Compared with the conventional strategy, we brought two acceptor units in para-relationship to each other on the phenothiazine backbone. Noticeably, emission was shifted hypsochromically to 430 nm in compound 3 from 480 nm, as observed for compound 2. Theoretical studies also provided a deep insight into the excitation and emission properties of the studied compounds. Frontier molecular orbitals and natural transition orbitals revealed that the extent of conjugation in compound 3 was much limited compared to 2. Single crystal X-ray studies helped to predict the packing modes of these compounds in relation to the substitution pattern. The butterfly angle in phenothiazine was found to be the lowest in compound 3. The phenothiazine ring was found to attain a plane perpendicular to the plane of the rest of molecule in 3 which was responsible to delimit the intermolecular stacking at the molecular level. The solution processed devices gave external quantum efficiencies of 2.7% and 1.6% with Commission Internationale de L'Eclairage (CIE) coordinates of 0.16, 0.13 and 0.16, 0.09 for devices using 2 and 3 as the dopant in the host matrix respectively. These results indicate that the introduction of acceptors in para-relationship on the phenothiazine core is a promising design strategy towards deep blue emitters.

 Please wait while we load your content...
Please wait while we load your content...