Distinct phosphorescence enhancement of red-emitting iridium(iii) complexes with formyl-functionalized phenylpyridine ligands†
Abstract
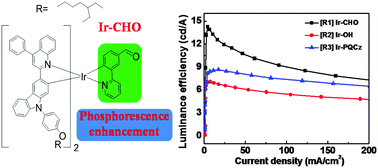
A new highly efficient red-emitting Ir(III) complex, bis [9-(4-(2-ethylhexyloxy)phenyl)-3-(4-phenylquinolin-2-yl)-9H-carbazole] [3-(pyridin-2-yl) benzaldehyde]iridium(III) (Ir-CHO), has been synthesized and characterized to investigate the impact of the electron-withdrawing formyl group on the optoelectronic properties of the resulting Ir(III) complexes. For comparison, bis [9-(4-(2-ethylhexyloxy)phenyl)-3-(4-phenylquinolin-2-yl)-9H-carbazole] [(3-(pyridin-2-yl) phenyl) methanol] iridium(III) (Ir-OH) and fac-tris[9-(4-(2-ethylhexyloxy)phenyl)-3-(4-phenylquinolin-2-yl)-9H-carbazole] iridium(III) (Ir-PQCz) were also prepared. Remarkably, Ir-CHO-doped red organic light-emitting diodes (OLEDs) exhibited the highest performance, achieving a maximum luminance efficiency (LE) of 14.3 cd A−1 and an external quantum efficiency (EQE) of 8.4%, which were better than those of Ir-OH-doped and Ir-PQCz-doped red OLEDs. The results can be attributed to the introduction of the electron-withdrawing formyl group, which improved the electron affinity in Ir-CHO and lowered the electron-injection barrier in OLEDs based on Ir-CHO. Similarly, white OLEDs (WOLEDs) with Ir-CHO as their red emitter demonstrated an excellent high LE of 26.1 cd A−1 and an EQE of 10.9%. A combination of the thermal, photophysical, electrochemical and electroluminescence (EL) properties of these Ir(III) complexes can thus lead to a conclusion that the incorporation of the electron-withdrawing formyl-substituted phenylpyridine (ppy-CHO) can apparently improve the phosphorescence of the resulting red-emitting iridium complexes and thus the OLED performance. The results suggest a simple methodology for the rational design of efficient phosphorescent complexes for light-emitting applications.

 Please wait while we load your content...
Please wait while we load your content...