Monodisperse macromolecules based on benzodithiophene and diketopyrrolopyrrole with strong NIR absorption and high mobility†
Abstract
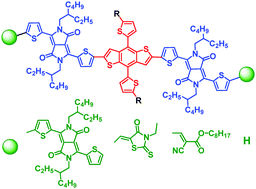
A series of monodisperse macromolecules with A2–A1–D–A1–A2 structure based on benzodithiophene (BDT) and diketopyrrolopyrrole (DPP) BDT-4DPP, BDT-DPP-Rhod and BDT-DPP-CA were designed, theoretically calculated and synthesized, and compared with their parent molecules BDT-2DPP and BDTS-2DPP with A1–D–A1 structure. These molecules possessed highly planar molecular geometries and high crystallinity. These molecules exhibited good thermal stability with decomposition temperatures of 322–388 °C, strong visible and near-infrared absorption (500–1000 nm), and HOMO energy levels of −5.38 to −5.19 eV and LUMO energy levels of −3.69 to −3.46 eV. Relative to the parent molecules A1–D–A1, A2–A1–D–A1–A2 molecules exhibited red-shifted and stronger absorption. The charge transport properties of these molecules were investigated by organic field-effect transistors, and their hole mobilities were 0.036–1.12 cm−2 V−1 s−1. Replacing alkyl with alkylthio on BDT led to mobility enhancement by one order of magnitude.
- This article is part of the themed collection: Small Molecules and Monodisperse Oligomers for Organic Electronics

 Please wait while we load your content...
Please wait while we load your content...