Three-dimensional hyaluronic acid hydrogel-based models for in vitro human iPSC-derived NPC culture and differentiation†
Abstract
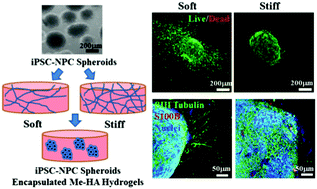
Human induced pluripotent stem cell-derived neural progenitor cells (hiPSC-NPCs) are considered as a promising cell source for transplantation and have been used for organoid fabrication to recapitulate central nervous system (CNS) diseases in vitro. The establishment of a three-dimensional (3D) in vitro model with hiPSC-NPCs and control of their differentiation are significantly critical for understanding biological processes and CNS disease and regeneration. Here we implemented 3D methacrylated hyaluronic acid (Me-HA) hydrogels with encapsulation of hiPSC-NPCs as in vitro culture models and further investigated the role of the hydrogel rigidity in the cell behavior of hiPSC-NPCs. We first encapsulated single dispersive hiPSC-NPCs within both soft and stiff Me-HA hydrogels and found that hiPSC-NPCs gradually self-assembled and aggregated to form 3D spheroids. Then, the hiPSC-NPCs were laden into Me-HA hydrogels in the form of spheroids to evaluate their spontaneous differentiation in response to hydrogel rigidity. The soft Me-HA hydrogel-encapsulated hiPSC-NPCs displayed robust neurite outgrowth and showed high levels of spontaneous neural differentiation. We further encapsulated Down Syndrome (DS) patient-specific hiPSC-derived NPC (DS-NPC) spheroids within our hydrogels. DS-NPCs retained excellent cell viability in both soft and stiff Me-HA hydrogels. Similarly, soft hydrogels promoted neural differentiation of DS-NPCs by significantly upregulating neural maturation markers. This study demonstrates that the soft matrix promotes neural differentiation of hiPSC-NPCs and HA-based hydrogels with hiPSC-NPCs or DS-NPCs are effective 3D models for a CNS disease study.


 Please wait while we load your content...
Please wait while we load your content...