Battery-like supercapacitors from diamond networks and water-soluble redox electrolytes†
Abstract
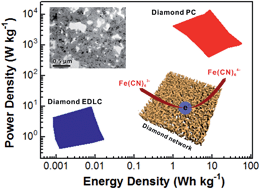
Enhanced performance of electrochemical capacitors can be achieved by larger capacitances as well as higher power and energy densities. In this work, such battery-like supercapacitors were fabricated using a three-dimensional and conductive diamond network as the capacitor electrode and water-soluble redox couples as the electrolyte. In 0.05 M Fe(CN)63−/4− + 1.0 M Na2SO4 aqueous solution, a capacitance of 73.42 mF cm−2 was obtained at a current density of 1 mA cm−2. This value is 10 000 times higher than the capacitance of diamond electric double layer capacitors (EDLCs). The energy and power densities of a fabricated diamond network symmetric pseudocapacitor were up to 56.50 W h kg−1 and 13.7 kW kg−1, respectively. Compared with those of diamond EDLCs obtained with the same cell voltage, they are enhanced about 3500 and 1440 fold, respectively. Therefore the combination of diamond networks and water-soluble redox electrolytes is a novel approach to construct electrochemical capacitors and thus bridges the gap between normal dielectric capacitors and rechargeable batteries.


 Please wait while we load your content...
Please wait while we load your content...