Challenges and prospects of the role of solid electrolytes in the revitalization of lithium metal batteries
Abstract
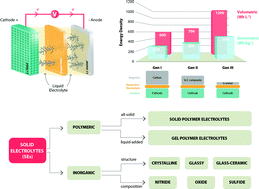
The scientific community is continuously committed to the search for new high energy electrochemical storage devices. In this regard, lithium metal batteries, due to their very high electrochemical energy storage capacity, appear to be a highly appealing choice. Unfortunately, the use of lithium metal as the anode may lead to some safety hazards due to its uneven deposition upon charging, resulting in dendrite growth and eventual shorting of the battery. This issue may be successfully addressed by using intrinsically safer electrolytes capable of establishing a physical barrier at the electrode interface. The most promising candidates are solid electrolytes, either polymeric or inorganic. The main purpose of this review is to describe the present status of worldwide research on these electrolyte materials together with a critical discussion of their transport properties and compatibility with metallic lithium, hoping to provide some general guidelines for the development of innovative and safe lithium metal batteries.


 Please wait while we load your content...
Please wait while we load your content...