A cost-effective synthesis of heteroatom-doped porous carbons as efficient CO2 sorbents†
Abstract
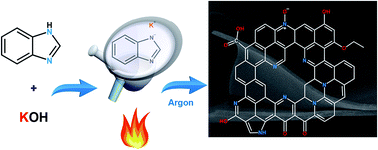
Carbon dioxide release into the atmosphere from fossil fuel burning has been identified as the major reason behind global warming. This has triggered immense interest in developing cost-efficient methods and materials for CO2 capture in recent years. In this study, benzimidazole was used as an inexpensive and commercially available single source precursor for carbon and nitrogen to successfully produce heteroatom-doped porous carbons (BIDCs) with remarkable CO2 capture properties. Our synthetic protocol involves solid-state addition of potassium hydroxide to the benzimidazole precursor to prevent its evaporation and subsequent carbonization–activation of the nonvolatile powdery mixture to ensure simultaneous porosity formation and heteroatom doping. The porous structure and heteroatom doping level can be controlled by tuning the KOH/benzimidazole ratio and carbonization temperature. The highest CO2 uptake values at 0.1 bar (1.60 mmol g−1), 1 bar (5.46 mmol g−1), and 30 bar (28.10 mmol g−1) were realized for three different carbons at 298 K. These diverse CO2 capture performances originate from a unique combination of surface chemistry and porous architecture for each BIDC. The carbon materials are regenerable and highly promising for CO2 separation or storage.

 Please wait while we load your content...
Please wait while we load your content...