Morphologically and compositionally tuned lithium silicate nanorods as high-performance carbon dioxide sorbents†
Abstract
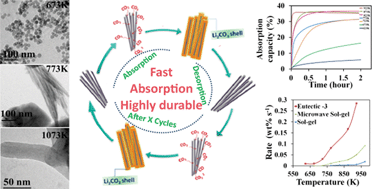
The effective capturing of carbon dioxide using regenerable high capacity sorbents is a prerequisite for industrial applications aiming at CO2 capture and sequestration. The removal of CO2 directly from chemical reaction environments at high temperature is a less energy intensive method of its separation with the added benefit of improved efficiency in equilibrium limited reactions. However, the separation of CO2 at the typical reaction temperatures of 573–1073 K is a challenging task due to the non-availability of absorbents with kinetics comparable to reaction rates. Moreover their poor durability due to sintering and particle growth on prolonged use at high temperature is also an impediment to their practical application. Herein, we demonstrate the development of an efficient CO2 absorbent material, made of Li4SiO4 nanorods, with ultrafast sorption kinetics as well as remarkable durability. These nanorods enabled easier surface reaction with CO2 due to shorter diffusion pathways for lithium from the bulk to the surface of the rods permitting extremely fast absorption of CO2. Furthermore, the compositional tuning of the materials helped to realize absorbents with extraordinary CO2 absorption rates of 0.72 wt% s−1 at 100% CO2/923 K. The exceptional performance of these absorbents at lower temperatures (573–823 K) as well as lower CO2 pressures (0.15 atm) demonstrates their potential in practical CO2 separation applications.


 Please wait while we load your content...
Please wait while we load your content...