Dehydrating bronze iron fluoride as a high capacity conversion cathode for lithium batteries†
Abstract
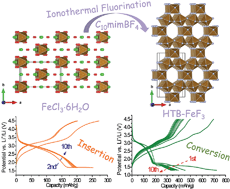
Lithium metal batteries combined with conversion cathodes are receiving more attention in view of their higher energy density. Open-framework fluoride is expected to have better conversion efficiency and reversibility than the dense polymorph, potentially benefitting from the optimized spatial distribution of its conversion products. However, the ease of hydration of the open channels would degrade the electrolyte when the framework evolves and hydration water is released during the conversion reaction. Herein, we report an ionothermal fluorination method to prepare dehydrated iron fluoride with a hexagonal tungsten bronze (HTB) structure using a microphase-separation-type ionic liquid for the first time. This bronze fluoride is characterized by high crystallinity and no substantial surface coating, which is crucial for the successful removal of tunnel H2O molecules. As a conversion cathode, it achieves a high reversible capacity of 200–450 mA h g−1 for at least 100 cycles, about 100 mA h g−1 larger than that for the hydrated HTB phase.

 Please wait while we load your content...
Please wait while we load your content...