Preparation and characterization of Sm and Ca co-doped ceria–La0.6Sr0.4Co0.2Fe0.8O3−δ semiconductor–ionic composites for electrolyte-layer-free fuel cells†
Abstract
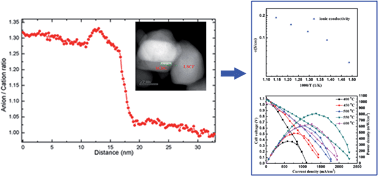
A series of Sm and Ca co-doped ceria, i.e. Ca0.04Ce0.96−xSmxO2−δ (x = 0, 0.09, 0.16, and 0.24) (SCDC), were synthesized by a co-precipitation method. Detailed morphology, composition, crystal structure and electrochemical properties of the prepared materials were characterized. The results revealed that Sm and Ca co-doping could enhance the ionic conductivity in comparison with that of single Ca-doped samples. The composition as Ca0.04Ce0.80Sm0.16O2−δ exhibited a highest ionic conductivity of 0.039 S cm−1 at 600 °C in comparison with the rest of the series, and the optimal ionic conductivity can be interpreted by the coupling effect of oxygen vacancies and mismatch between the dopant ionic radius and critical radius. Composite formation between the semiconductor La0.6Sr0.4Co0.2Fe0.8O3−δ (LSCF) and the as-prepared SCDC contributed to a remarkable improvement in the ionic conductivity, an unexpectedly high ionic conductivity of 0.188 S cm−1 was obtained for LSCF–SCDC composites at 600 °C, which was four times higher than that of pure SCDC. Using transmission electron microscopy and spectroscopy approaches, we detected an enrichment of oxygen in the LSCF–SCDC interface region and a depletion of oxygen vacancies in LSCF–SCDC and LSCF–LSCF grain boundaries was significantly mitigated, which resulted in the enhancement of ionic conductivity of semiconductor–ionic LSCF–SCDC composites. The electrolyte-layer-free fuel cell (EFFC) fabricated from the LSCF–SCDC semiconductor–ionic membrane demonstrated excellent performances, e.g. 814 mW cm−2 at 550 °C for using the LSCF–Ca0.04Ce0.80Sm0.16O2−δ (SCDC2).

 Please wait while we load your content...
Please wait while we load your content...