Guanidinium nonaflate as a solid-state proton conductor†
Abstract
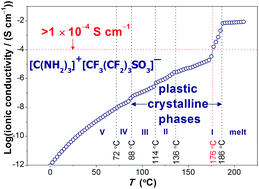
Protic organic ionic plastic crystals (POIPCs) are a type of novel solid-state proton conductors. In this work, guanidinium nonaflate ([Gdm-H][NfO]) is reported to be a model POIPC. Its structure–property relationship has been investigated comprehensively. Infrared analysis of [Gdm-H][NfO] and its deuterated analogue [Gdm-D][NfO] confirms the complete formation of the protic salts. The cations in as-prepared [Gdm-D][NfO] are estimated to consist of [C(ND2)2(NHD)]+ and [C(ND2)3]+ with a molar ratio of around 1 : 1. The deuteration also proves that each guanidinium cation has six displaceable protons. Thermogravimetric analysis demonstrates that [Gdm-H][NfO] exhibits superior thermal stability in both nitrogen and air atmospheres. Isothermogravimetric analysis reveals its negligible vapor pressure with an estimated high enthalpy of vaporization (120.9 kJ mol−1). Differential scanning calorimetry measurements of [Gdm-H][NfO] show four evident endothermic peaks prior to its melting transition at 186.2 °C with a low entropy of melting (17.70 J K−1 mol−1). Shortly before the onset temperature of melting transition (186.2 °C), partial melting (partial liquefaction) was observed via polarized optical microscopy in the temperature region of 176–186 °C while the reason for partial melting of ionic plastic crystals is not clear yet. Variable-temperature powder X-ray diffraction tests confirm the related solid-solid phase transitions and demonstrate that [Gdm-H][NfO] exhibits short-range disorder and long-range positional order in the plastic crystalline phases. Dielectric spectroscopy measurements show that its ionic conductivity reaches 2.1 × 10−3 S cm−1 at 185 °C. The proton conduction in the plastic crystalline phases of [Gdm-H][NfO] is assumed to happen via the vehicle mechanism. In the molten state, the proton conduction follows a combination of the vehicle mechanism and the Grotthuss mechanism (structural diffusion). In summary, due to their exceptional physicochemical properties, POIPCs like [Gdm-H][NfO] are promising electrolyte materials for high temperature (100–200 °C) proton exchange membrane fuel cells. In addition, POIPC-based solid-state proton conductors are also expected to find applications in sensors and other electrochemical devices.


 Please wait while we load your content...
Please wait while we load your content...