Interfacial reaction-directed synthesis of a ceria nanotube-embedded ultra-small Pt nanoparticle catalyst with high catalytic activity and thermal stability†
Abstract
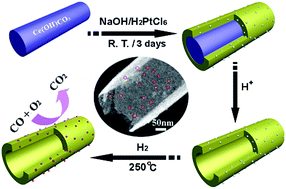
A catalyst based on ceria nanotube-embedded ultra-small Pt nanoparticles was synthesized by means of an interfacial reaction in the absence of any surfactant and without involving any separate surface modification process. When Ce(OH)CO3 nanorods and H2PtCl6 are introduced into a NaOH aqueous solution in sequence, a solid–liquid interfacial reaction between Ce(OH)CO3 and NaOH occurs. The formed Ce(OH)3 then deposits on the external surface of Ce(OH)CO3 nanorods. During the interfacial reaction, the negatively charged Pt species is expected to be electrostatically attracted to gradually formed Ce(OH)3 due to its positive charge, resulting in a uniform mixture of Pt species and Ce(OH)3. After removing residual Ce(OH)CO3 and hydrogen reduction, ceria nanotube-embedded Pt nanoparticle hollow composites were achieved. Due to the ultra-small size of catalytically active Pt nanoparticles and the close contact between Pt and ceria, the catalyst exhibits high catalytic activity toward CO oxidation and excellent thermal stability even at temperatures as high as 700 °C, suggesting that they also hold promise for higher temperature catalytic reactions.

 Please wait while we load your content...
Please wait while we load your content...