3DOM-LaSrCoFeO6−δ as a highly active catalyst for the thermal and photothermal reduction of CO2 with H2O to CH4†
Abstract
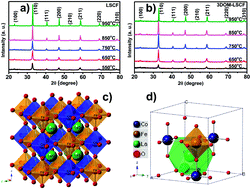
Double perovskites LaSrCoFeO6−δ (LSCF) and LaSrCoFeO6−δ with a three-dimensionally ordered macroporous structure (3DOM-LSCF) were successfully synthesized by a facile combustion process. Their crystal structure, morphology, BET surface area, band gap and catalytic properties were characterized in detail. Phase pure double perovskites LSCF and 3DOM-LSCF can be obtained by calcination at 550–950 °C for 4 h. The ordered and interconnected pore structure generated by using a PMMA template can be maintained successfully in the 3DOM-LSCF catalyst. Both catalysts had good catalytic performance in either CH4 selectivity or total yield. Production of CH4 from CO2 and H2O can reach 351.32 μmol g−1 for LSCF and 557.88 μmol g−1 for 3DOM-LSCF under photothermal conditions (350 °C + vis-light) in 8 h. The high solar-to-methane (STM) energy conversion efficiency was 1.217% for LSCF and 1.933% for 3DOM-LSCF in the photothermal mode. The results also show that the yield of CH4 in the photothermal mode is 5 times that in the thermal reduction mode. The double perovskites LSCF and 3DOM-LSCF are promising catalytic materials for the photothermal reduction of CO2 to hydrocarbon fuels.

 Please wait while we load your content...
Please wait while we load your content...