Synergetic effects of edge formation and sulfur doping on the catalytic activity of a graphene-based catalyst for the oxygen reduction reaction†
Abstract
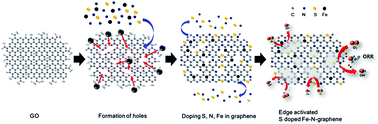
An edge activated S doped Fe-N-graphene (EA-SFeNG) was successfully synthesized via facile ball milling followed by a pyrolysis process. The oxygen reduction reaction (ORR) performance of EA-SFeNG was dramatically improved by doping S and forming edge sites in Fe-N-graphene; the onset potential was shifted from 0.91 VRHE to 1.0 VRHE with the half-wave potential increased from 0.77 VRHE to 0.848 VRHE. The EA-SFeNG exhibited catalytic performances that are comparable to those of commercial 20 wt% Pt/C (Vonset: 1.05 V, V1/2: 0.865 V); however, its durability was better than that of the Pt catalyst in alkaline media. The excellent ORR activity can be attributed to the increase in defect density and SOx bonding in the EA-SFeNG. Furthermore, we experimentally demonstrate that the work function of the Fe-N-graphene is significantly reduced from 4.06 eV to 4.01 eV by the increase in edge density and doping S, thereby improving the ORR kinetics of EA-SFeNG.

 Please wait while we load your content...
Please wait while we load your content...