Equipping an adsorbent with an indicator: a novel composite to simultaneously detect and remove heavy metals from water†
Abstract
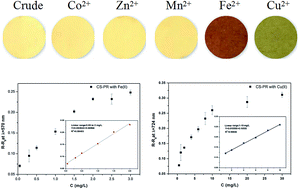
A novel composite that can simultaneously detect and remove heavy metals was prepared in this study. Its adsorption ability rivals that of excellent traditional adsorbents; furthermore, it can change the color to identify the adsorbate and its concentration. The color changes are very easy to detect via the naked eye or with a photometer. This novel adsorbent can remove a large amount of heavy metal ions, and also qualitatively and quantitatively detect heavy metal ions in water. Cu(II) and Fe(II) were used to investigate the detection and removal properties of the composite, and SEM, BET, FTIR, zeta potential and XPS were used to physicochemically characterize it. Batch sorption experiments were conducted to study the effects of various factors such as pH, the presence of coexisting metal ions, the contact time and the initial metal concentration. The studies indicated that the adsorption process fits well to the Langmuir model, and the kinetic data could be described very well by the pseudo-second-order kinetic model. The mechanism of adsorption has been discussed in detail. This study provides a simple method for the preparation of smart adsorbents.

 Please wait while we load your content...
Please wait while we load your content...