Well-dispersed and porous FeP@C nanoplates with stable and ultrafast lithium storage performance through conversion reaction mechanism†
Abstract
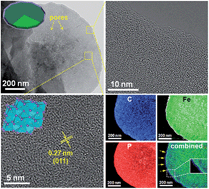
Conformally carbon-coated FeP (FeP@C) nanoplates with a porous feature are synthesized by integrating three simple approaches: hydrothermal growth of Fe2O3 nanoplates, the spatial introduction of conformal carbon coating, and chemical conversion of an intermediate into FeP by a low-temperature phosphidation strategy. The introduction of carbon coating not only serves as a buttering to effectively prevent the restacking of nanoplates, but also largely improves electrical conductivity and reinforces the structural robustness of FeP. The obtained FeP@C nanoplates show two-dimensional morphology and possess a large surface-area-to-volume-ratio and abundant inner mesopores with a high specific surface area. As an anode of lithium-ion batteries (LIBs), the prepared FeP@C nanoplates exhibit exceptionally high electrochemical performance, which largely outperforms the Fe3O4@C counterpart under identical morphological properties in experimental and simulated results. As a result, the FeP@C nanoplates exhibit a reversible specific capacity of 720 mA h g−1 and a capacity retention rate of 96% after 100 cycles at a current density of 200 mA g−1. Even at a higher current density of 500 mA g−1, it still delivers a stable capacity of 610 mA h g−1 within 400 cycles with negligible capacity fading, which opens venues for long life-time applications of LIBs. Furthermore, the superior rate capability of such anodes is also obtained with a reversible specific capacity of 347 mA h g−1 at 5000 mA g−1. The outstanding lithium ion storage properties of the FeP@C nanoplates may be attributed to the reduced energy barrier for the Li storage reaction, shortened electronic/ionic transfer paths, and the physical buffering effect rooted in the rational integration of the phosphide-based species, plate-like nanostructure and conformal carbon surface modification.

 Please wait while we load your content...
Please wait while we load your content...