The cross-substitution effect of tantalum on the visible-light-driven water oxidation activity of BaNbO2N crystals grown directly by an NH3-assisted flux method†
Abstract
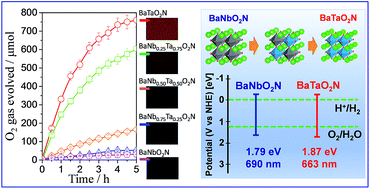
Various perovskite-type transition metal oxynitride photocatalysts have been intensively investigated for photocatalytic water splitting under visible light. The band gap engineering and the formation of solid solution have been demonstrated to be one of the beneficial approaches to achieve a high solar energy conversion efficiency. As a member of the niobium-based oxynitride family, BaNbO2N has a small band gap energy of 1.79 eV and light absorption up to 690 nm. Here, we have investigated the cross-substitution effect of tantalum on the photocatalytic water oxidation activity of BaNbO2N crystals grown directly by an NH3-assisted flux method. It was found that with increasing the amount of tantalum substituted for niobium in BaNb1−xTaxO2N (x = 0, 0.25, 0.50, 0.75, and 1), the average crystal size and the intensity of background absorption gradually reduced and the O2 evolution rate increased monotonically for BaTaO2N crystals due to the shift of the top of the valence band to a more positive side and the lowered densities of mid-gap states associated with defects. The CoOx/flux-grown-BaTaO2N/Ta photoanode exhibited a photocurrent of about 0.85 mA cm−2 at 1.2 VRHE, which decreased gradually by about 35% after 24 h, evidencing that the flux-grown BaTaO2N crystals are highly stable for photoelectrochemical water oxidation. BaTaO2N modified with CoOx (2 wt% Co) as an O2 evolution cocatalyst exhibited an apparent quantum yield (AQY) of 0.24% at 420 nm for an O2 evolution reaction in the presence of Ag+ ions as sacrificial electron acceptors. In addition, the results from the first-principles DFT calculations conform to the experimentally obtained data on the photocatalytic water oxidation activity of the BaNb1−xTaxO2N crystals.


 Please wait while we load your content...
Please wait while we load your content...