Reproducible magnetic carbon nanocomposites derived from polystyrene with superior tetrabromobisphenol A adsorption performance†
Abstract
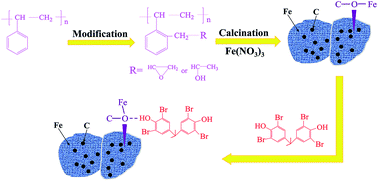
Easily reproducible magnetic carbon nanoadsorbents (MPSNs) derived from polystyrene (PS) by direct calcination of functionalized PS with an iron salt are employed for effective tetrabromobisphenol A (TBBPA) adsorption. Batch adsorption tests indicate that MPSNs exhibited high adsorption affinity to aqueous TBBPA with a maximum adsorption capacity of 117.00 mg g−1. The TBBPA adsorption kinetics of MPSNs are found to obey the pseudo-second-order behavior with a calculated room temperature initial adsorption rate of 42.871 mg g−1 min−1 for the solution with an initial TBBPA concentration of 4.0 mg L−1 and pH value of 8.0. Monolayer adsorption of the Langmuir isotherm model is well fitted rather than the multilayer adsorption of the Freundlich isotherm model. The calculated thermodynamic parameters suggest that the TBBPA adsorption on MPSNs is spontaneous and exothermic. The optimal pH value for TBBPA adsorption on MPSNs is around 8.0 with an MPSNs dose of 30.0 mg L−1 and contact time of 30 min under sonication at 298 K. The TBBPA adsorption performance of MPSNs is strongly influenced by the presence of humic acid. The prepared MPSN retains around 85.5% of TBBPA adsorption capacity after 5 cycles and exhibits good reusability. The explored adsorption mechanism by Raman and Fourier transform infrared (FT-IR) spectroscopy suggests that the C–O–Fe bond in the MPSN is responsible for the superior TBBPA adsorption performance. This work provides promising magnetic adsorbents for TBBPA wastewater treatment as well as a new strategy to recycle and reuse polymer plastic waste.

 Please wait while we load your content...
Please wait while we load your content...