Carbon–boron core–shell microspheres for the oxygen reduction reaction†
Abstract
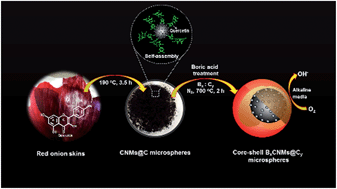
Core–shell carbon nanomaterials (CNMs) with high oxygen reduction reaction (ORR) efficiency and greater stability have become potential alternatives to the expensive Pt/C used in direct methanol fuel cells (DMFCs). Here, we demonstrate a simple strategy for the preparation of electroactive metal-free catalysts from wastes (red onion skins) for improving ORR efficiency. Through hydrogen bonding and π–π stacking interactions between/among aromatic rings of the molecules, many CNMs with sizes mostly smaller than 20 nm are self-assembled on the surface of each carbon microsphere having an average diameter of 0.9 ± 0.1 μm. To further improve the electrocatalytic activity of CNMs@C microspheres, they have been subjected to treatment with boric acid, leading to the formation of a boron (B) shell on each CNMs@C microsphere. The B0.5CNMs@C1.0 electrode exhibits higher catalytic activity than the CNMs@C electrode, with advantages of high stability and tolerance against methanol and CO poisoning.


 Please wait while we load your content...
Please wait while we load your content...