Enhanced conversion reaction kinetics in low crystallinity SnO2/CNT anodes for Na-ion batteries†
Abstract
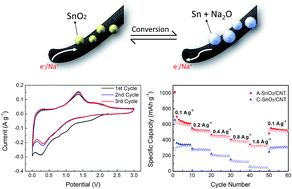
The specific capacities of SnO2 anodes in sodium ion batteries (SIBs) are far below the values expected from theory. Herein, we propose that the kinetically-controlled, reversible ‘conversion reaction’ between Na ions and SnO2 is responsible for Na ion storage in SnO2 anodes where the ion diffusion rate is the limiting factor. This revelation is contrary to the current understanding of the ‘alloying reaction’ as the major reaction process. Aiming to fully utilize the theoretical capacity from the conversion reaction, a composite electrode consisting of carbon nanotubes coated with a mainly amorphous SnO2 phase together with crystalline nanoparticles is synthesized. The SnO2/CNT anodes deliver a superior specific capacity of 630.4 mA h g−1 at 0.1 A g−1 and 324.1 mA h g−1 at a high rate of 1.6 A g−1 due to the enhanced kinetics. The volume expansion of the composite is accommodated by the CNT substrate, giving rise to an excellent 69% capacity retention after 300 cycles. The aforementioned findings give new insight into the fundamental understanding of the electrochemical kinetics of SnO2 electrodes and offer a potential solution to the low capacity and poor cyclic stability of other metal oxide anodes based on conversion reactions.

 Please wait while we load your content...
Please wait while we load your content...