Manganese dioxide nanosheet functionalized sulfur@PEDOT core–shell nanospheres for advanced lithium–sulfur batteries†
Abstract
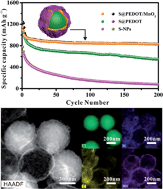
Lithium–sulfur (Li–S) batteries are receiving significant attention as an alternative power system for advanced electronic devices because of their high theoretical capacity and energy density. In this work, we have designed manganese dioxide (MnO2) nanosheet functionalized sulfur@poly(3,4-ethylenedioxythiophene) core–shell nanospheres (S@PEDOT/MnO2) for high performance lithium–sulfur (Li–S) batteries. A PEDOT layer is used to address the low electrical conductivity of sulfur and acts as a protective layer to prevent dissolution of polysulfides. The MnO2 nanosheets functionalized on PEDOT further provide a high active contact area to enhance the wettability of the electrode materials with electrolytes and further interlink the polymer chains to improve the conductivity and stability of the composite. As a result, S@PEDOT/MnO2 exhibits an improved capacity of 827 mA h g−1 after 200 cycles at 0.2C (1C = 1673 mA g−1) and a further ∼50% enhancement compared to S@PEDOT (551 mA h g−1) without MnO2 functionalization. In particular, the discharge capacity of S@PEDOT/MnO2 is 545 mA h g−1 after 200 cycles at 0.5C. Our demonstration here indicates that the functionalization of inorganic nanostructures on conducting polymer coated sulfur nanoparticles is an effective strategy to improve the electrochemical cycling performance and stability of sulfur cathodes for Li–S batteries.

 Please wait while we load your content...
Please wait while we load your content...