NiFe2O4–CNT composite: an efficient electrocatalyst for oxygen evolution reactions in Li–O2 batteries guided by computations†
Abstract
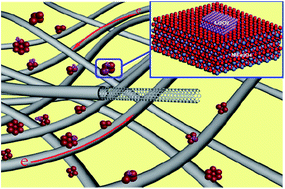
Lithium–oxygen batteries are regarded as the most promising candidate for future energy storage systems. However, their poor rechargeability and low efficiency remain critical barriers for practical applications. By using first-principles computations, we disclosed that NiFe2O4 has superior oxygen evolution reaction (OER) activity for the decomposition of Li2O2. Guided by computations, we prepared a composite of NiFe2O4 and carbon nanotubes (CNTs) through a hydrothermal route and applied it to Li–O2 batteries. The batteries with NiFe2O4–CNT air cathodes displayed lower charging overpotential and better cycling performance than those with CNT air cathodes. The improved electrochemical performance was attributed to the high OER activity of NiFe2O4 for the decomposition of Li2O2.


 Please wait while we load your content...
Please wait while we load your content...