Design and synthesis of energetic materials towards high density and positive oxygen balance by N-dinitromethyl functionalization of nitroazoles†
Abstract
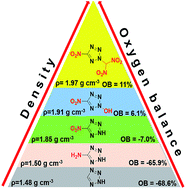
A new N-functionalized strategy of nitrogen heterocycles was utilized for the synthesis of nitroazole-based energetic materials, giving rise to a new family of highly dense and oxygen-rich energetic materials. They were characterized by IR spectroscopy, NMR spectroscopy, elemental analysis, DSC, and X-ray diffraction. These new molecules exhibit high densities, moderate to good thermal stabilities, acceptable impact and friction sensitivities, and excellent detonation properties, which suggest potential applications as energetic materials or oxidizers. Interestingly, among tetrazole-based CHNO energetic materials compound 5 has the highest measured density of 1.97 g cm−3 to date. 5c is the first and the only heterocyclic CHNO energetic salt with a positive OB until now. Compounds 5 and 6 exhibit excellent detonation properties (38.5 GPa, 9.22 km s−1; 37.0 GPa, 9.05 km s−1), comparable to the highly explosive HMX. With high OB, the specific impulses of 5, 5b, 5c, and 6c are superior to those of AP and ADN as neat compounds, and the ratio of oxidizer/aluminium/PBAN (%) is 80 : 20 : 0 or 80 : 13 : 7. Furthermore, computational results, BDEs, Mulliken charges and Wiberg bond orders also support the superior qualities of the newly prepared compounds and the design strategy.

 Please wait while we load your content...
Please wait while we load your content...