Nanoporous amide networks based on tetraphenyladamantane for selective CO2 capture†
Abstract
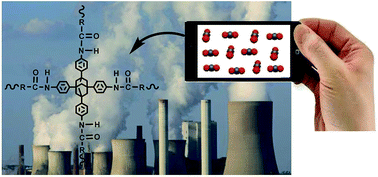
Reduction of anthropogenic CO2 emissions and CO2 separation from post-combustion flue gases are among the imperative issues in the spotlight at present. Hence, it is highly desirable to develop efficient adsorbents for mitigating climate change with possible energy savings. Here, we report the design of a facile one pot catalyst-free synthetic protocol for the generation of three different nitrogen rich nanoporous amide networks (NANs) based on tetraphenyladamantane. Besides the porous architecture, CO2 capturing potential and high thermal stability, these NANs possess notable CO2/N2 selectivity with reasonable retention while increasing the temperature from 273 K to 298 K. The quantum chemical calculations also suggest that CO2 interacts mainly in the region of polar amide groups (–CONH–) present in NANs and this interaction is much stronger than that with N2 thus leading to better selectivity and affirming them as promising contenders for efficient gas separation.

 Please wait while we load your content...
Please wait while we load your content...