Experimental and theoretical studies on competitive adsorption of aromatic compounds on reduced graphene oxides†
Abstract
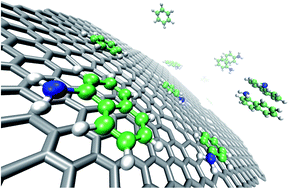
The individual and competitive adsorption studies of benzene, aniline and naphthylamine on reduced graphene oxides (rGOs) were investigated by batch experiments and theoretical density functional theory (DFT). Experimental results indicate that (1) in all the single, binary, and ternary aromatic compound systems, the sequence of maximum adsorption capacity is naphthylamine > aniline > benzene on rGOs; (2) the overall adsorption capacity of rGOs is in the order of ternary > binary > single system. The DFT calculations indicate that (1) the adsorption energy (Ead) follows the order of Ead (benzene) < Ead (aniline) < Ead (naphthylamine); (2) the binding energy (Ebd) values of aromatic mixtures indicate that the intra-molecular interactions between the aromatic compounds themselves have an important influence on their adsorption on rGOs. The DFT calculations are in good agreement with the batch adsorption results. These findings are very important and useful to understand the mechanisms of adsorption of aromatic compounds on rGOs as well as assessing the effect of the benzene-ring number and polar functional groups on the adsorption of coexisting aromatic compounds on rGOs. The contents are important for the application of rGOs in environmental pollution management.

 Please wait while we load your content...
Please wait while we load your content...