Unique walnut-shaped porous MnO2/C nanospheres with enhanced reaction kinetics for lithium storage with high capacity and superior rate capability†
Abstract
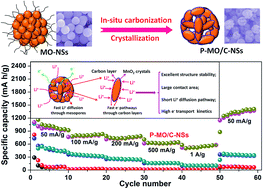
Unique walnut-shaped porous MnO2/carbon nanospheres (P-MO/C-NSs) with high monodispersity have been designed and prepared for lithium storage via in situ carbonization of amorphous MnO2 nanospheres. Polyvinylpyrrolidone (PVP) is utilized as both the surfactant for morphology control and carbon source for carbon scaffold formation accompanied with MnO2 crystallization. Such a unique walnut-shaped porous nanostructure with an intimate carbon layer provides a large contact area with the electrolyte, short transport path length for Li+, low resistance for charge transfer and superior structural stability. The P-MO/C-NS electrode demonstrates high lithium storage capacity (1176 mA h g−1 at 100 mA g−1), very good cycling stability (100% capacity retention versus the second cycle) and excellent rate capability (540 mA h g−1 at 1000 mA g−1). We propose that it is the deep oxidation of Mn2+ to Mn3+ in P-MO/C-NSs, which results in an extraordinarily high capacity of 1192 mA h g−1 at a current density of 1000 mA g−1 after a long period of cycling, very close to the maximum theoretical reversible capacity of MnO2 (1230 mA h g−1). This is the highest value ever observed for MnO2-based electrodes at such a rate. The high lithium storage capacity and rate capability can be attributed to the enhanced reaction kinetics owing to the walnut-shaped porous nanostructure with an intimate carbon layer. This work provides a meaningful demonstration of designing porous nanostructures of carbon-coated metal oxides undergoing deep conversion reactions for enhanced electrochemical performances.

 Please wait while we load your content...
Please wait while we load your content...