Highly efficient dye-sensitized solar cells based on a ruthenium sensitizer bearing a hexylthiophene modified terpyridine ligand†
Abstract
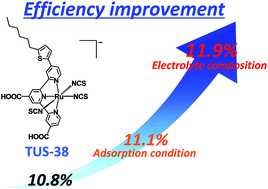
Two ruthenium sensitizers bearing a 4-substituted terpyridine derivative ligand (TUS-39 and TUS-41) have been synthesized for dye-sensitized solar cells (DSCs). Solar cell performances of DSCs with these TUS sensitizers, together with that of the DSC with TUS-38 which is a structurally analogous ruthenium sensitizer bearing an n-hexylthiophene modified terpyridine derivative ligand, have been evaluated to investigate a favorable substituent for improving the photosensitization ability of ruthenium sensitizers. Among the three TUS sensitizers, TUS-38 showed the highest conversion efficiency of 10.8% under AM 1.5 (100 mW cm−2) irradiation. The conversion efficiency of the DSC with TUS-38 can be increased to 11.1% by decreasing the dye-adsorption temperature from 20 °C to 4 °C, presumably due to the improvement in the molecular arrangement of the sensitizers adsorbed at the TiO2 surface. This improved conversion efficiency can be further enhanced to 11.9% by employing an electrolyte solution containing 1-ethyl-3-methylimidazolium iodide instead of conventional 1,2-dimethyl-3-n-propylimidazolium iodide. This conversion efficiency is considerably higher than that of Black dye (10.9%), which is one of the most famous and highly efficient ruthenium sensitizers, under the same conditions. Upon optimization of the dye-adsorption conditions and the electrolyte composition, an extremely high conversion efficiency of 11.9% can be obtained for the DSC with TUS-38.

 Please wait while we load your content...
Please wait while we load your content...