Rational design of a novel indole-based microporous organic polymer: enhanced carbon dioxide uptake via local dipole–π interactions†
Abstract
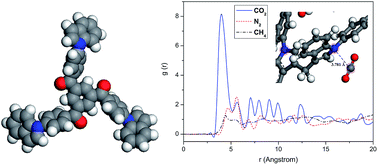
An indole-based microporous organic polymer (PINK) has been obtained by the condensation polymerization of 1,3,5-tris-(4-fluorobenzoyl)benzene with 3,3′-diindolylmethane via a catalyst-free nucleophilic substitution reaction. Due to the local dipole–π interactions between indole and carbon dioxide (CO2), the uptake capacity for CO2 reaches up to 16.0 wt% (1.0 bar, 273 K), and the high (CO2/N2 = 15, CO2/CH4 = 32) selectivities of the polymer make it a promising material for potential application in gas separation. Furthermore, the hydrogen storage is up to 2.48 wt% (1.0 bar, 77 K). In comparison to the reported porous organic polymers, the preparative strategy exhibits cost-effective advantages, which are essential for scale-up preparation. Its good performance for H2 storage and CO2 separation suggests that PINK with a large specific surface area shows potential use in clean energy applications and the environmental field.

 Please wait while we load your content...
Please wait while we load your content...