Adsorption and desorption behavior of ionic and nonionic surfactants on polymer surfaces†
Abstract
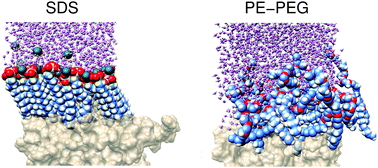
We report combined experimental and computational studies aiming to elucidate the adsorption properties of ionic and nonionic surfactants on hydrophobic polymer surface such as poly(styrene). To represent these two types of surfactants, we choose sodium dodecyl sulfate and poly(ethylene glycol)–poly(ethylene) block copolymers, both commonly utilized in emulsion polymerization. By applying quartz crystal microbalance with dissipation monitoring we find that the non-ionic surfactants are desorbed from the poly(styrene) surface slower, and at low surfactant concentrations they adsorb with stronger energy, than the ionic surfactant. If fact, from molecular dynamics simulations we obtain that the effective attractive force of these nonionic surfactants to the surface increases with the decrease of their concentration, whereas, the ionic surfactant exhibits mildly the opposite trend. We argue that the difference in this contrasting behavior stems from the physico-chemical properties of the head group. Ionic surfactants characterized by small and strongly hydrophilic head groups form an ordered self-assembled structure at the interface whereas, non-ionic surfactants with long and weakly hydrophilic head groups, which are also characterized by low persistence lengths, generate a disordered layer. Consequently, upon an increase in concentration, the layer formed by the nonionic surfactants prevents the aprotic poly(ethylene glycol) head groups to satisfy all their hydrogen bonds capabilities. As a response, water molecules intrude this surfactant layer and partially compensate for the missing interactions, however, at the expense of their ability to form hydrogen bonds as in bulk. This loss of hydrogen bonds, either of the head groups or of the intruding water molecules, is the reason the nonionic surfactants weaken their effective attraction to the interface with the increase in concentration.

 Please wait while we load your content...
Please wait while we load your content...