Modelling and predicting the interactions between oppositely and variously charged polyelectrolytes by frontal analysis continuous capillary electrophoresis†
Abstract
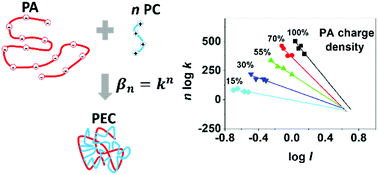
In this work, a systematic study of the interactions between poly(L-lysine) and variously charged statistical copolymers of acrylamide and 2-acrylamido-2-methyl-1-propanesulfonate (PAMAMPS) has been carried out by frontal analysis continuous capillary electrophoresis (FACCE). FACCE was successfully implemented to obtain the interaction parameters (binding constant and stoichiometry) at different ionic strengths and for different PAMAMPS charge densities varying between 15% and 100%. The range of investigated ionic strengths was carefully adjusted according to the PAMAMPS charge density to obtain measurable binding constants by FACCE (i.e. formation binding constant typically comprised between 104 and 106 M−1). The number of released counter-ions during the polyelectrolyte complex formation was systematically quantified via the ionic strength dependence of the binding constant and was compared to the total condensed counter-ion reservoir according to Manning theory on counter-ion condensation. A descriptive and predictive model relating the physico-chemical properties of the two partners, the binding constant and the ionic strength is proposed in the framework of multiple independent interaction sites of equal energy.

 Please wait while we load your content...
Please wait while we load your content...