Solvent effects on the fracture of chemically crosslinked gels
Abstract
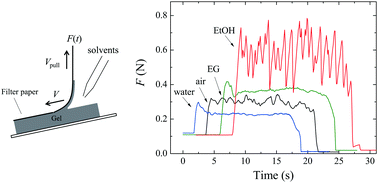
We have investigated how the fracture behavior of a polyacrylamide hydrogel is affected by different types of solvents poured into its crack tips. We obtained the following results: first, when water (good solvent or reaction solvent for the polyacrylamide gel) is poured, the fracture energy Γ becomes smaller than that measured in air for small crack velocities V (V ≤ 10 mm s−1). Second, when good solvents other than water are poured, Γ is enhanced for a large V region (5 ≤ V ≤ 60 mm s−1), but this effect is not observed for smaller V; Γ(V) in good solvents converges to that in water as V → 0. Third, when ethanol (poor solvent for polyacrylamide) is poured, stick–slip-like crack propagation appears in the entire V range, and Γ calculated from the time-average of the oscillating tearing forces is larger than that in air or in other solvents. We discuss the results on the basis of diffusion dynamics around the crack tips of the gel.

 Please wait while we load your content...
Please wait while we load your content...