Dimeric peptides with three different linkers self-assemble with phospholipids to form peptide nanodiscs that stabilize membrane proteins
Abstract
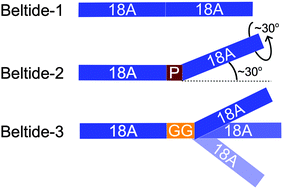
Three dimers of the amphipathic α-helical peptide 18A have been synthesized with different interhelical linkers inserted between the two copies of 18A. The dimeric peptides were denoted ‘beltides’ where Beltide-1 refers to the 18A-dimer without a linker, Beltide-2 is the 18A-dimer with proline (Pro) as a linker and Beltide-3 is the 18A-dimer linked by two glycines (Gly–Gly). The self-assembly of the beltides with the phospholipid DMPC was studied with and without the incorporated membrane protein bacteriorhodopsin (bR) through a combination of coarse-grained MD simulations, size-exclusion chromatography (SEC), circular dichroism (CD) spectroscopy, small-angle scattering (SAS), static light scattering (SLS) and UV-Vis spectroscopy. For all three beltides, MD and combined small-angle X-ray and -neutron scattering were consistent with a disc structure composed by a phospholipid bilayer surrounded by a belt of peptides and with a total disc diameter of approximately 10 nm. CD confirmed that all three beltides were α-helical in the free form and with DMPC. However, as shown by SEC the different interhelical linkers clearly led to different properties of the beltides. Beltide-3, with the Gly–Gly linker, was very adaptable such that peptide nanodiscs could be formed for a broad range of different peptide to lipid stoichiometries and therefore also possible disc-sizes. On the other hand, both Beltide-2 with the Pro linker and Beltide-1 without a linker were less adaptable and would only form discs of certain peptide to lipid stoichiometries. SLS revealed that the structural stability of the formed peptide nanodiscs was also highly affected by the linkers and it was found that Beltide-1 gave more stable discs than the other two beltides. With respect to membrane protein stabilization, each of the three beltides in combination with DMPC stabilizes the seven-helix transmembrane protein bacteriorhodopsin significantly better than the detergent octyl glucoside, but no significant difference was observed between the three beltides. We conclude that adaptability, size, and structural stability can be tuned by changing the interhelical linker while maintaining the properties of the discs with respect to membrane protein stabilization.


 Please wait while we load your content...
Please wait while we load your content...