Solvent effects on modulus of poly(propylene oxide)-based organogels as measured by cavitation rheology†
Abstract
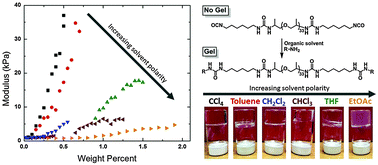
A series of novel organogels were synthesized from poly(propylene oxide) (PPO) functionalized with main chain urea moieties which provided rapid gelation and high moduli in a variety of solvents. Three different molecular weight PPOs were used in this study: 430, 2000, and 4000 g mol−1, each with α,ω-amino-end groups. Four urea groups were introduced into the main chain by reaction with hexamethylene diisocyanate followed by subsequent reaction with a monofunctional alkyl or aromatic amine. This PPO/urea gelator was found to form gels in carbon tetrachloride, chloroform, dichloromethane, toluene, ethyl acetate, and tetrahydrofuran. Among these, carbon tetrachloride and toluene were found to be the best solvents for this system, resulting in perfectly clear gels with high moduli at low mass fraction for PPO-2000 systems. Flory–Huggins polymer–solvent interaction parameter, χ, was found to be a useful indicator of gel quality for these systems, with χCCl4/PPO-2000 < 0.5 and χtoluene/PPO-2000 ≈ 0.5. Systems with χ parameters >0.5 were found to form low moduli gels, indicating that for these systems, polymer–solvent interaction parameters can be a useful predictor of gel quality in different solvent systems.


 Please wait while we load your content...
Please wait while we load your content...