Fluorophore exchange kinetics in block copolymer micelles with varying solvent–fluorophore and solvent–polymer interactions†
Abstract
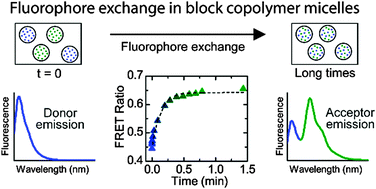
Fluorescence spectroscopy was employed to characterize the kinetics of guest exchange in diblock copolymer micelles composed of poly(ethylene oxide-b-ε-caprolactone) (PEO–PCL) diblock copolymers in water/tetrahydrofuran (THF) mixtures which encapsulated fluorophores. The solvent composition (THF content) of the micelle solution was varied as a means of modulating the strength of interactions between the fluorophore and solvent as well as between the micelle core and solvent. A donor–acceptor fluorophore pair was employed consisting of 3,3′-dioctadecyloxacarbocyanine perchlorate (DiO, the donor) and 1,1′-dioctadecyl-3,3,3′,3′-tetramethylindocarbocyanine perchlorate (DiI, the acceptor). Through the process of Förster resonance energy transfer (FRET), energy was transferred from the donor to acceptor when the fluorophores were in close proximity. A micelle solution containing DiO was mixed with a micelle solution containing DiI at t = 0, and the emission spectra of the mixed solution were monitored over time (at an excitation wavelength optimized for the donor). In micelle solutions containing 5 and 10 vol% THF in the bulk solvent, an increase in the acceptor peak intensity maximum occurred over time in the post-mixed solution, accompanied by a decrease in the donor peak intensity maximum, indicating the presence of energy transfer from the donor to the acceptor. At long times, the FRET ratios (acceptor peak intensity divided by the sum of the acceptor and donor peak intensities) were indistinguishable from that determined from pre-mixed micelle solutions of the same THF content (in pre-mixed solutions, DiO and DiI were encapsulated within the same micelle cores). In the micelle solution containing 20 vol% THF, the fluorophore exchange process occurred too quickly to be observed (the FRET ratios measured from the solutions mixed at t = 0 were commensurate to that measured from the pre-mixed solution). A time constant describing the guest exchange process was extracted from the time-dependence of the FRET ratio through fit of an exponential decay. An increase in the THF content in the micelle solution resulted in a decrease in the time constant, and the time constant varied over five orders of magnitude as the THF content was varied from 5–20 vol%.

 Please wait while we load your content...
Please wait while we load your content...